

About Us
Singular Focus,
Passionate Team
Our team has deep expertise that spans the entire drug development timeline from concept to clinical trial to commercialization, specific to cardiopulmonary disease. We are excited to advance novel therapies focused on providing meaningful improvements for people living with this debilitating disease.
Our Approach
Targeting
Disease Drivers
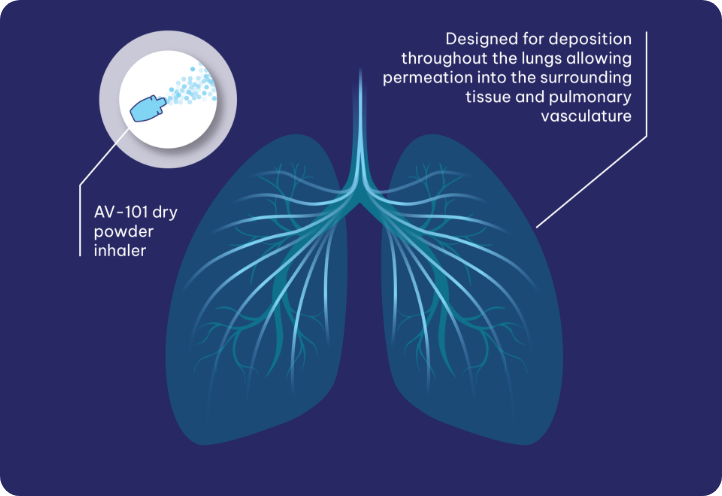
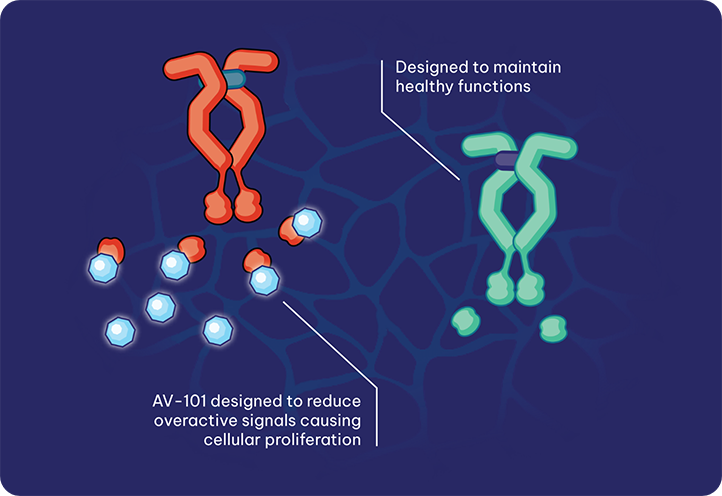
Pulmonary Arterial Hypertension (PAH) is a rare progressive and often fatal cardiopulmonary disease affecting both the heart and lungs. While conventional therapeutics may help improve disease symptoms, they do little to address the underlying drivers of disease. Our lead program, AV-101, an investigational, proprietary dry powder inhaled formulation of imatinib, is intended to address the underlying hyperproliferation of cells within the narrowed arteries of the lungs.




Join Our Team
One Goal, One Purpose
At Aerovate, we are committed to developing new therapies that vastly improve the lives of people living with rare cardiopulmonary disease. We’ve assembled an experienced team of problem-solvers and cardiopulmonary disease experts to take us from drug development through commercialization.
We need dynamic, purpose-driven and motivated innovators like you. Learn more about how your unique skills and talents can make a difference at Aerovate.